Recent content from Joe Carlson

Concerns grow over magnets in common gadgets disabling medical devices
Following reports about iPhone 12, researchers are investigating a variety of gadgets containing magnets.

Minnesota defends N.D. laws on drug-benefit companies
State AG Ellison says states have the right to regulate pharmacy-benefit companies.

Mayo Clinic faces lawsuits over nude patient image snooping
A former surgery resident faces criminal charges in the 2020 matter, and three lawsuits contend Mayo is responsible.

Boston Sci to add to its heart-device portfolio
Medical device maker to buy California firm in $450 million deal
Hospitals, nursing homes not mandating vaccine — yet
After a closely watched legal victory for a Texas health system, no hospital or nursing home in Minnesota so far has imposed a COVID-19 vaccine mandate on its workers.

Medtronic pulls heart-failure device off the market
Following reports of greater mortality and stroke risk, Medtronic pulls its HVAD device from worldwide market.

Many Minnesotans choose to stay masked after mandate's end
The risk of public encounters with the unmasked and unvaccinated is still a concern, especially for those with children too young to be vaccinated.

NAMSA in growth mode under new leadership
Recent changes in ownership, executive leadership signal moves for near-term expansion.

Minnesota hospitals offering more N95 masks to workers
Minnesota hospitals collectively now have about 2.8 million N95 respirators on hand, a supply that would last five months. A year ago, they had only enough to last two months, state data show.

Minnesota extends workers' comp for first responders hit by COVID-19
The benefit was set to end May 1, but Gov. Tim Walz signed an extension through year's end.

Nursing home worker's death triggers record COVID fine
Sholom Community Alliance paid $27,100 in fines after David Kolleh, a manager in the facility's memory care unit, died from the coronavirus as it spread in the home.

Minnesota settles lawsuit with New Prague tavern that violated virus restrictions
The Scott County bar continued to serve food and drinks indoors despite state orders prohibiting that.

Minnesota launches mobile vaccine clinics by bus
Outreach focused on groups hit hard by COVID-19 including communities of color.

Intensive care for COVID-19 rising in Minnesota, despite flat trend in overall hospitalizations
1,189 new COVID-19 cases, five deaths in Minnesota

Questions surface over state's COVID-19 testing contract
Under the emergency agreement struck last year, the state is billed $87 to $120.99 per test, depending on whether the sample came from a free community walk-up site or the statewide mail-order testing program.
HealthPartners, Allina partnership aims to lower costs by making care more effective
Allina patients insured by HealthPartners will be included in five-year effort focused on improving outcomes.

Medtronic warns of potential battery problem in 340,000 defibrillators
The device maker is not recommending that doctors remove the devices unless they see a sign of problems.

Some COVID-19 long haulers in Minnesota still lack diagnosis
There's no ready pool of doctors with the necessary expertise who can quarterback the complicated issues faced by the silent wave of Long COVID patients. Patients say it feels like they're on their own.

UnitedHealth's proposed $13 billion IT acquisition gets closer regulatory review
A national hospital group is concerned the deal could distort patient care decisions and claims processing.

Minnesota farms, food industry face another pandemic growing season
Last year the COVID-19 pandemic triggered major disruptions in the state's $112 billion industry. Meatpacking plant closures and declines in sales to restaurants forced some livestock to be euthanized.
Options are expanding for rapid at-home COVID testing
Minnesota is one of 10 states getting federal shipments of the Cue Health test, which delivers results in minutes. But it's not free.
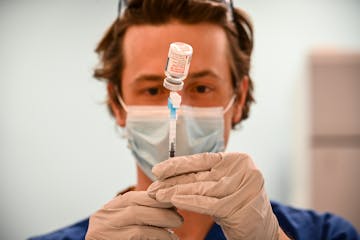
As vaccine options grow, some get picky in Minnesota
Despite public health advice that people should get whatever vaccine is first available to them, some vaccine-seekers are using online tools to locate a specific brand at a retail site. Some public sites also list vaccine availability by brand.

'Heart in a Box' device allows more transplants in Minnesota
Table-sized device works as life support during transplant.

Boston Scientific to pay $1.1B for kidney stone laser system
The med-tech company said the deal is expected to close in the second half of 2021.

Testing remains important even as Minnesotans get vaccinated
When COVID-19 case counts fall, doctors and public health officials say the importance of finding and isolating asymptomatic carriers will re-emerge as a top priority for ending the pandemic.

No workers' comp paid for COVID-19 claims so far at Minnesota meatpacking plants
None of the claims that have been paid so far are from the state's largest meat-processing plants, where some of the Minnesota's biggest workplace outbreaks occurred.
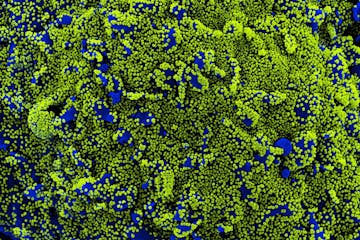
Scientists look to Twin Cities sewers to find COVID variants
Minnesota has seen 18 cases of the U.K. variant, two cases of the Brazil variant, and no cases of the South Africa variant, according to CDC data.

Workplace COVID-19 complaints flood state safety agency
Minnesota saw 250% increase over prior year.

Companies still struggling to make enough pandemic supplies
N95 respirators. Rubber gloves. Rapid test kits and swabs. Special syringes. To boost production, the Biden administration plans to use a wartime law that prioritizes federal contracts.

Pandemic takes bite out of state's health care employment
The number of people working in health care — Minnesota's largest industry by worker head count — has dropped 10,000 since the start of the year.

COVID test used across state may offer clues about new strain
The manufacturer of a test used at community testing sites across the state says the test may offer an important clue that a more infectious COVID strain is present.

Goodbye nasal swabs: Minnesota expands saliva testing capacity
Starting this month, the Minnesota Department of Health is eliminating nasal-swab testing at its 20 "barrier-free" COVID-19 testing sites, and moving to collecting only saliva samples. The state's at-home tests will remain saliva-based.

As pandemic year winds down, mental health concerns rise in Minn.
Seen as the pandemic's possible "next wave"; experts offer ways to help.

Company will surrender counterfeit N95 masks to be destroyed
Supply Link officials said they were conducting an investigation to determine who was behind the fake masks.

As COVID-19 vaccines roll out, facemasks will still be essential
Scientists don't yet know of vaccinated people can spread the virus, how fast immunity might fade or whether booster shots will be needed after the initial two-injection course of the vaccine.
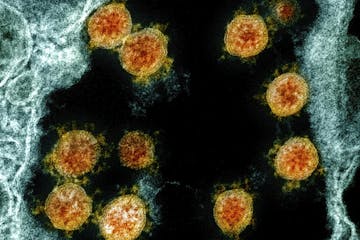
New test by Minnesota biotech firm can detect COVID immunity levels
Available to the public for the first time, it adds word to virus vocabulary: titer.

Antidepressant may keep COVID patients out of the hospital
An anxiety-fighting drug that has been on the market for decades could turn out to be a potent weapon in the fight against COVID-19.

Demand for COVID-19 tests in Minn. surges ahead of holidays
Demand is so strong in Minnesota that Health Department officials are modifying their prior advice for asymptomatic people to get tested.

Boston Scientific plans to cut 106 jobs at Twin Cities plant
Decision to drop heart valve means 100 jobs lost at Maple Grove plant.

State's COVID-19 cases soaring as families make Thanksgiving plans
Family get-togethers seen as high risk as deaths in Minnesota exceed 2,900.

6 things you need to know about COVID-19 testing in Minnesota
Which COVID test works best? Is it free? Where can I get one? We spoke to medical experts to answer these questions and more.
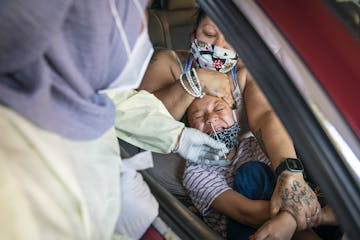
Quarantined health workers feel pressure to return to work
As COVID-19 cases in Minnesota continue to surge, some hospitals and health care providers are asking employees with "higher-risk" exposure to the disease to return to work before their quarantines end.
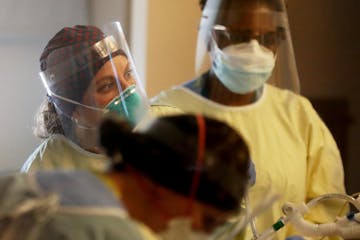
Virus caregivers have more risk at home, in community, study finds
Bedside caregivers are far less likely to catch COVID-19 after risky exposures to patients, compared with interactions at home or in the community, new data show.

Rural Minnesota provider Sanford Health merging with system based in Salt Lake City
Health system would join with Intermountain, a similar hospital network.
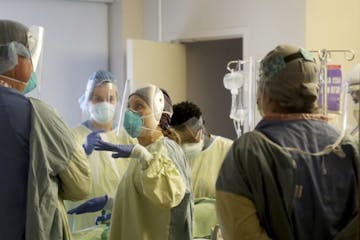
COVID makes heart attacks more deadly, study finds
"Mortality for heart attack patients ... should be in single digits. We're seeing mortality here that is 32%," said Dr. Santiago Garcia, of the Minneapolis Heart Institute Foundation.

Medtronic tells investors it expects faster sales growth ahead
The company now expects to return to normal revenue and profit growth by January, three months earlier than earlier projections.
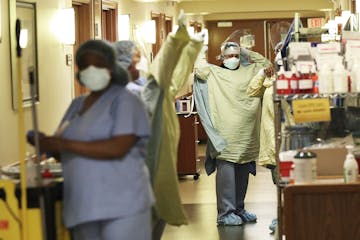
Health workers losing pay over testing delays, quarantine rules
A bill slated to be introduced in the Legislature on Monday would require hospitals and nursing homes to give paid time off to health care workers who need to go on leave for COVID testing and quarantine.

Former Medtronic CEO Omar Ishrak stepping down from company's board
Omar Ishrak, currently executive chairman, will separate from device maker in December.

Former Mayo Clinic employee improperly accessed 1,600-plus patient health records
Social Security numbers, payment card information and bank account numbers weren't accessed.

Medtronic 'cooperating fully' as U.S. investigates ventilator competition
The Department of Justice is looking into whether Medtronic's acquisitions led to suppressed competition in the market.
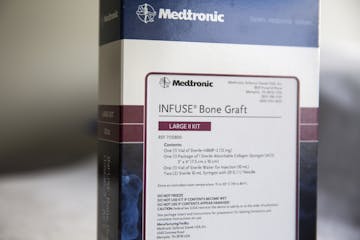
Medtronic product removed from market in Australia, which is now investigating its use
Medtronic denies any wrongdoing in its marketing or sales of Infuse.

Minnesota schools grapple with shifts in COVID-19 cases
Despite state guidelines to help school districts decide whether or when to switch to hybrid or full-time remote learning models, the recommendations are nonbinding, leaving it to district administrators to make the final call.

U testing dorm sewage for COVID-19 at Twin Cities, Duluth campuses
The University of Arizona and Utah State University recently quarantined and tested hundreds of students after dorm wastewater samples led to the discovery of undetected COVID-19 cases.

State's second-largest health care data breach hits Children's, Allina
The hack was part of a ransomware attack on a cloud computing company called Blackbaud, which manages databases for a number of nonprofits.
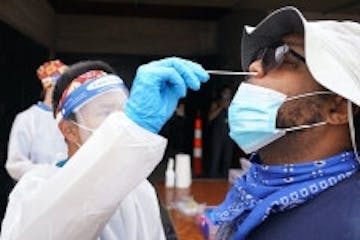
COVID widens racial gap in Minnesota's health care system
As hospitals and clinics rebuild from the pandemic's financial hit, some doctors and health care officials say there's never been a better time to reach out to historically underserved communities and offer them the same levels of care as whites.
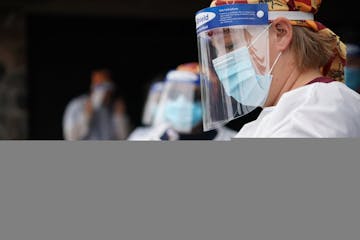
N95 masks rationed in state, six months into pandemic
"They are still rationing them, just like they were back in March," said one nurse at a metro hospital, who is leaving her "dream job" until the pandemic recedes because she doesn't feel safe.

Pioneering transplant surgeon Dr. John Najarian dies at 92
He served for decades as head of surgery at the University of Minnesota, in a career marked by achievement and controversy.

Mayo puts Rochester skyscraper expansion on hold 'indefinitely'
Gonda Building would have been Rochester's tallest tower.

Two Twin Cities hospitals hit with penalties over COVID-19
North Memorial and United Hospital were each hit with $2,100 citations after workers complained to the state about an array of allegedly unsafe practices related to breathing devices and other personal protective equipment.

With second virus wave coming, state waits for millions of N95 masks
Masks ordered, but getting them is another story.

Medtronic hopes to expand share of diabetes management market with new acquisition
Companion Medical will help the company expand its diabetes management offerings.

United Hospital faces lawsuit over safety after firing ER nurse
Cliff Willmeng joins a burgeoning group of hospital workers nationally filing lawsuits in response to what they see as pressure from hospitals to unreasonably lower safety standards for workers on the front lines of pandemic care.
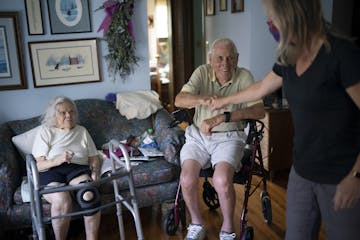
How North Memorial is helping non-COVID patients do more at home
Hospital-at-home programs are providing telehealth for some emergency patients
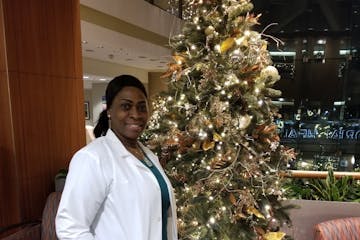
COVID death of North Memorial worker prompts OSHA probe
The president of Larrydean Goodridge's union said that after her death, the hospital reversed its policy of not providing non-nursing staff with N95 respirators. Hospital officials say they were following CDC guidelines.
'Return to work' COVID antibody testing comes with warning
Health officials say the evidence backing test accuracy and protectiveness from antibodies is not yet strong enough. Even the lab companies and hospitals admit they can't offer "immunity certificates" to people who have the antibodies today.

Minnesota cities set their own mask rules to fight COVID-19
Elected officials say requiring cloth masks while in public is an easy and safe to way prevent spread of the coronavirus.

Uptick in ICU numbers, confirmed COVID-19 cases continues in Minnesota
Seven of the eight people whose deaths were reported Friday lived in long-term care facilities.
![A social distancing sign at Lake Harriet Thursday afternoon. ] aaron.lavinsky@startribune.com The scene at Lake Harriet's South Beach photographed Thu](https://arc.stimg.co/startribunemedia/ITSX3NW2ZGBXHFSJGH7G7DPPMQ.jpg?h=120&w=180&fit=crop&bg=999&crop=faces)
Minnesotans, nation prepare to celebrate July 4th with COVID-19 cases on the rise
Health Commissioner Jan Malcolm urged Minnesotans to celebrate the holiday primarily with the people in their own households and to consider virtual visits with larger groups.

500 new cases, 13 deaths in COVID-19 pandemic in Minnesota
It's only the third report in a month's time to reach the 500s.

Walz is urged to mandate masks in Minnesota in COVID-19 fight
State health officials say evidence is mounting that masks make a difference in slowing COVID-19 spread.

Hospitalizations for COVID-19 continue to decline in Minnesota
Gov. Tim Walz has said the number of new COVID-19 cases confirmed by laboratory testing in Minnesota plateaued in June.